Solution
If a liquid is made of only one substance, it is called a pure liquid. If the liquid is made of two or more substances, it is called a mixture. A solution is a homogeneous mixture of two or more non–reacting substances A solution of two components is called a binary solution. When two substances form a solution, one substance is said to dissolve in the other substance. The component present in smaller proportion is called a solute and the component present in larger proportion is called a solvent
Solute + Solvent = Solution
Commonly found solutions contain liquid solvent. A solution is clear and trans- parent. For example, when sugar is dissolved in water, it forms a clear solution. There is no new substance formed. Hence formation of solution is a physical change. Water is a universal and polar solvent. Many solids dissolve in water. The solutions where water is used as a solvent, are called aqueous solutions. The solutions where liquids other than water are used as a solvent are called non–aqueous solutions and the solvents are called non–aqueous solvents. Benzene, chloroform, alcohol, carbon tetrachloride are some non–aqueous solvents.
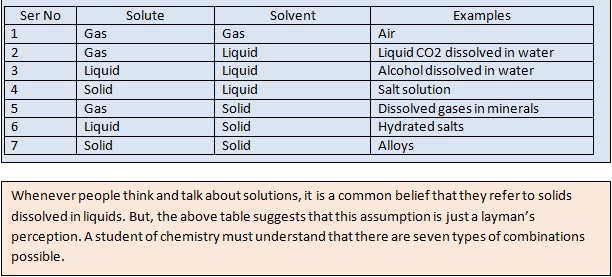
Types of Solutions
Based on the physical state of the components, following types of solutions are possible