Thomson’s model of atom –Discovery of the electron
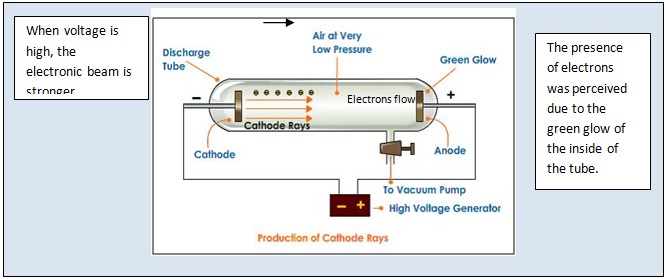
J. J. Thomson, in 1897, provided the first clue to the structure of atom. He carried out his experiment using a cathode ray tube. His idea was to break atoms of gases into small particles under high voltage in a discharge tube. The tube was essentially a glass tube that could contain gases at very low pressure. The cathode was a rod or a plate that was connected to the negative terminal of a battery or an electrical source. The anode was connected to a positive terminal of the battery. When a high voltage like 10,000 volts was applied across the two electrodes at ordinary pressure, surprisingly, no current passed through them. But when pressure inside the tube was reduced, the entire tube was filled with magenta–red type of glow with air inside the tube. Thomson observed that rays (particles) originated from cathode and went to the anode. Thomson called these particles as corpuscules which later came to be known as electrons. This experiment proved that atoms contain electrons. Thomson’s cathode ray tube is shown below
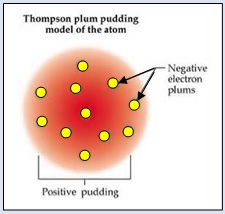
Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of a positive charge, were like currants (dry fruits) in a spherical Christmas pudding. We can also think of a water melon. The positive charge in the atom is spread all over like the red edible part of the water melon while the electrons are studded in the positively charged sphere, like the seeds in the water melon.