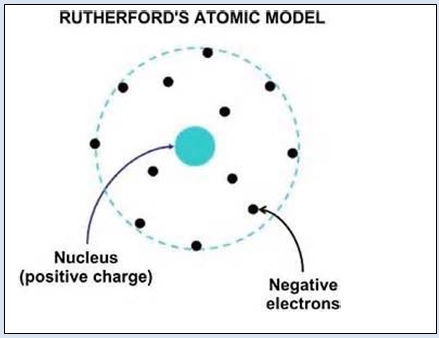
Rutherford’s model of atom
On the basis of his experiment, Rutherford put forward the nuclear model of an atom. It had following features.
- There is a positively charged center in an atom called nucleus. Nearly all the mass of the atom resides in the nucleus.
- The electrons revolve around the nucleus in well defined orbits.
- The number of electrons in all the orbits is equal to the number of positive charges ( protons ) in the nucleus of the atom. Hence every atom is electrically neutral.
- The size of the nucleus is very small as compared to the size of the atom.
Rutherford’s model of atom is shown below.
English
