Bohr’s model of atom
In order to overcome the objections raised against Rutherford’s model of atom , Niel Bohr, from Denmark, in 1913, put forward the postulates about the model of atom.
- Laws of mechanics and electrodynamics are not applicable to the structure of atom.
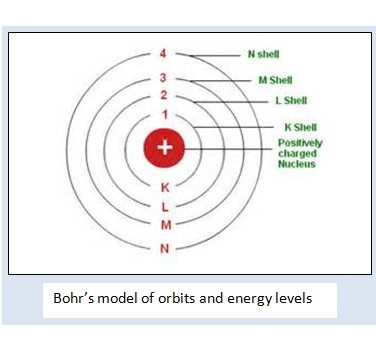
- Electron in the atoms rotate in discrete selected orbits or concentric cir- cular paths without radiating energy. These orbits are called stationary orbits or states. They are called K, L, M, N ......and numbered 1, 2, 3, 4 ......
-
Only those orbits are stationary which satisfy the condition m x v x r = n x h / 2π
Where h = Plank’s constant = 6.34 x 10−34 ergs sec. m = mass of the electron
v = velocity of the electron; r = radius of stationary orbit; n = number of the orbit = 1,2,3 ...... - Electron can jump from one stationary orbit to another stationary orbit by either absorbing or emitting energy bunch. The magnitude the energy is equal to the difference in energy of the two stationary orbits i.e. E2 –E1 = h ν
- The space between the two orbits is a forbidden area for the electrons.
English
