Double decomposition reactions
These reactions are also called ’Double Displacement Reactions.’ Let us consider following few examples.
Take a few ml dilute solution of barium chloride in a test tube. Add equal volume of dilute solution of sodium sulphate to it. Stir the resulting mixture with a glass rod. Barium chloride reacts with sodium sulphate to from barium sulphate and sodium chloride. Following figure shows the formation of barium sulphate and sodium chloride.
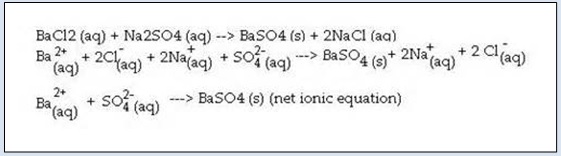
- In this reaction, two substances viz. BaCl2 and Na2 SO4 react to from two products viz. BaSO4 and NaCl. The cations Ba2+ and Na+ of the reactants exchange their partners Cl− and SO4 2− respectively with each other during the reaction.
Take a few ml of dilute solution of ammonium chloride in a test tube. Add equal volume of dilute solution of sodium hydroxide to it. Stir the reaction mixture with a glass rod. Sodium hydroxide reacts with ammonium chloride to form sodium chloride and ammonium hydroxide.
- In this reaction, two substances viz. NaOH and NH4Cl react to from two products viz. NaCl and NH4OH . The cations Na+ and NH4 +4 of the reactants exchange their partners OH− and Cl− respectively with each other during the reaction.
Take a few ml dilute solution of calcium chloride in a test tube. Add equal volume of dilute solution of ammonium carbonate to it. Stir the reaction mixture with a glass rod. Calcium chloride reacts with ammonium carbonate to form calcium carbonate and ammonium chloride.
- In this reaction, two substances viz. CaCl2 and (NH4)2 CO3 react to form two products viz. CaCO3 and NH4Cl . The cations Ca2+ and NH4 + of the reactants exchange their partners Cl− and CO3 2− respectively with each other during the reaction.
In the above examples, two reactants have combined to form two products.
A chemical reaction in which the constituents ( cations and anions ) of two compounds mutually exchange their places to form two new compounds is called a double decomposition reaction.
Double decomposition reactions usually take place between ionic compounds. These reactions are, sometimes, called double displacement reactions. Most of the precipitation reactions of ionic compounds come under the class of double decomposition reactions. Some more examples of double decomposition reactions are given below.
1. Ca(OH)2 + 2 NH4Cl → CaCl2 + 2 NH4OH
2. PbCl2 + 2 KI → PbI2 + 2 KCl
3. CuSO4 + H2S → CuS + H2 SO4
4. AgNO3 + KCl → AgCl + KNO3
