Electron configuration of atom
The energy of an electron depends upon he shell in which it is placed. Electron in the K shell has minimum energy and the energy goes on increasing as the electron occupies higher and higher energy shells i.e. K, L, M, N, etc. electron configuration is he arrangement or distribution of electrons in different shells.
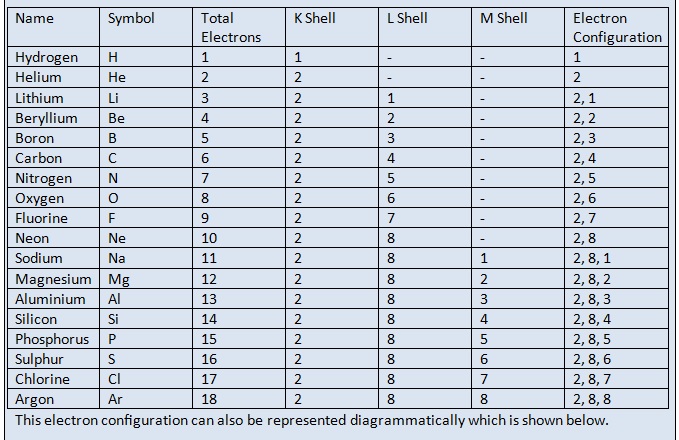
Electronic Configurations of Atoms
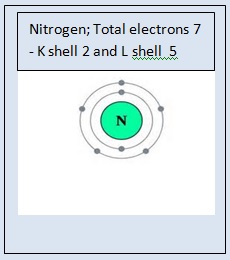
Electrons occupy the shells in the increasing order of energy. The electron configuration of nitrogen is written as 2,3 which means that nitrogen atom has 2 electrons in the first shell ( K shell ) and 3 electrons in the second shell ( or L shell ). The electron configuration of chlorine is written as 2, 8, 7 which means that chlorine atom has 2 electrons in the first shell( K shell ) , 8 electrons in the second shell ( L shell ) and 7 electrons in the third shell ( M shell ). The electron configuration of first 18 elements is shown in the following table.