RUTHERFORD’S SCATTERING EXPERIMENT AND ATOMIC MODEL
Thomson proposed that (i) an atom consists of a positively charge sphere and the electrons are embedded in it and (ii) the negative and positive charges are equal in magnitude, so the atom is neutral.
Later a German scientist, E. Goldstein made the discovery of protons.
Rutherford’s scattering experiment and atomic model
Rutherford, in 1911, carried out a scattering experiment to understand the in- ternal distribution of positive and negative charges in the atom. He bombarded a thin gold foil with very small positively charged particles called α –particles. The gold foil has a thickness of about 1000 atoms of gold. The α –particles were nothing but the positively charge helium atoms viz. He2+ . Since the mass of alpha particles 4 u, these fast moving particles have considerable energy. A simplified picture of his experiment is shown below.
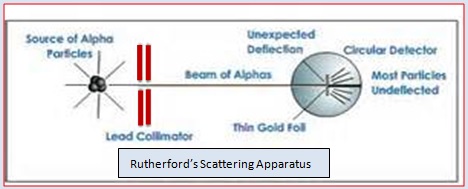
Rutherford observed that when α –particles strike the gold foil, most of them pass through the foil without any deflection from their path. But some particles show a little deflection while some particles show considerable deflection. He found that some particles completely rebound to their original path.
The above experiment can best be illustrated by a fallowing example. Let a child stand in front of a barbed –wire fence. Let him throw stones on the fence. Most of the stones would not hit the fence. This is because there are lot of gaps in the fence which allow the stones to pass through them. The stones which hit the wire may be deflected or may come back in the same direction.
From his experimental observations, Rutherford concluded that –
- Since a large number of α –particles passed through the atom without changing direction, there is a large empty space in the atom.
- Some of them were deflected back which shows that the positively charged α –particles meet some positively charged body in the nucleus of the atom. Since the number of such deflections are very small, it shows that the nucleus is very very small in size compared to the size of the atom.
- Deflection by large angle can be explained by saying that α –rays passing by the nucleus suffer repulsions due to like charges and are deflected away by large angles.
