Combination reactions
These are also called ’Synthesis Reactions’. Let us discuss following few examples.
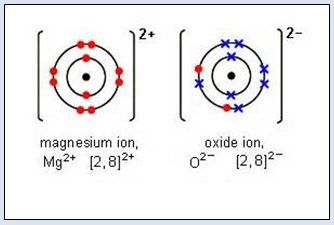
1. Take a magnesium ribbon and hold it in pair of tongs. Ignite the mag- nesium ribbon in a Bunsen burner flame. Magnesium ribbon burns with a dazzling white light and gives a white ash of magnesium oxide. Magnesium metal combines with oxygen to form magnesium oxide. This is shown in the following figure
2. Take a little amount of mercury in a hard glass tube. It has a silvery shining colour. Heat the tube slowly. The shining mercury changes into a reddish powder of mercuric oxide. Mercury combines with oxygen to form mercuric oxide.
3. Tale a glass rod and dip it in concentrated hydrochloric acid. Take out the rod and hold it immediately near the mouth of bottle of ammonia. Dense white fumes of ammonium chloride are produced. Hydrochloric acid reacts with ammonia to form ammonium chloride.
In the above examples, two substances (reactants) have combined to form a single product.
A chemical reaction in which two or more reactants combine to form a single product is called a combination or synthesis reaction.
The reactants taking part in the combination reaction can be elements or compounds or a combination of elements and compounds.
Some more examples of combination reactions are given below.
1. H2 + Cl2 → 2 HCl
2. CaO + H2O → Ca(OH)2
3. PCl3 + Cl2 → PCl5
4. 2SO2 + 2H2O + O2 → 2H2 SO4
.
